Effect of Fermented Corn Protein Concentrate on Growth Performance, Haemocyte Counts, Histological Structure of Hepatopancreas and Intestinal Condition of Pacific White Shrimp Litopenaeus Vannamei
Abstract: This study evaluated the suitability of commercially produced fermented corn protein concentrate (FCPC) known as Motiv™ (Cargill’s IP Product), in replacing fish meal (FM) and/or poultry by-product meal (PBM) on growth and health performances of the Pacific white shrimp Litopenaeus vannamei. A 60-day feeding trial was conducted to evaluate the use of five iso-nitrogenous and iso-lipidic diets containing 0% FCPC (control), 7.5% FCPC and 1.5% Krill meal (KM) replacing 9% of FM (FCPC 1), 7.5% FCPC and 1% KM replacing 2% poultry by-product meal (PBM) and 6.5% FM (FCPC2), 7.5% FCPC replacing 3.5% PBM and 4% FM (FCPC3) and 7.5% FCPC to replace 7.5% PBM (FCPC4) on growth performance, total haemocytes count, lysozyme activity, body composition, resistance on acute salinity change, total number of bacteria and histomorphological condition of the hepatopancreas of Pacific white shrimp Litopenaeus vannamei. At the end of the growth trial, the dietary treatments significantly affect the growth performance of shrimp with better growth obtained in shrimp treated with FCPC 1 and 4 compared to the control treatment. For the non-specific immune system, the inclusion of FCPC had significantly improved the lysozyme activity of L. vannamei but no significant effect to the total haemocytes counts. Total numbers of bacteria were lower in the hepatopancreas of shrimp fed on FCPC diets compared to the control treatment. In addition, FCPC diets increased survivability of shrimp acute salinity stress compared to the control group. Our findings suggested that FCPC could be used as an alternative for a sustainable approach to replace FM and/or PBM in shrimp diets to have better growth as well as enhancing stress resistances and increasing antibacterial responses of shrimp through enhancing the lysozyme activities in L. vannamei.
1 | Introduction
Nowadays, Pacific white shrimp Litopenaeus vannamei is one of the aquaculture commodities that has a significant increase in market demand, production growth and also has a fairly good level of profitability if maintained with proper management system (Ariadi et al., 2019; Karim et al., 2014; Martinez-Cordero & Leung, 2004; Rego et al., 2017). According to FAO (2020) production of L. vannamei accounted for 52.9% of crustacean aquaculture in 2018 and rapidly expanded from 2.64 million tons in 2010 to 4.96 million tons in 2018. Technically, the positive trend was also driven by technology, including the breeding technology (Argue et al., 2002; Wang et al., 2019; Xiong et al., 2011), development of biofloc and green water technology (Arantes et al., 2017; Chithambaran et al., 2017), feed management system (Reis et al., 2020; Silva et al., 2019), biosecurity technology (Lightner, 2005; Moss et al., 2012) and raise awareness about sustainability in shrimp production system (Lebel et al., 2010; Lebel et al., 2016). All these technologies and innovations could optimise the seed quality, growth, survival rates and culture environments of L. vannamei, thus reducing uncertainty in the production system.
As shrimp production continues to grow, so does the requirement for sustainable practical diet that could have beneficial impacts on growth and intestinal health of shrimp but do not deplete the natural resources (Colombo & Turchini, 2021; Jackson, 2012; Novriadi & Davis, 2018). Historically, FM has been considered as the preferred protein source for the aquafeed because of its nutrient characteristics as the excellent source of essential amino acid, fatty acid, vitamins, mineral, palatability and lack of anti-nutritional factors (ANFs) (Davis & Arnold, 2000). However, concerns on environmental sustainability, biodiversity and natural resources have pushed the industry and scientists in innovation and development of FM alternatives allowing increased levels of plant-based proteins (Lim & Dominy, 1990; Sookying et al., 2013), including the co-products from corn milling industry (Guo et al., 2019; Qiu et al., 2017; Rhodes et al., 2015; Sookying & Davis, 2011). The use of co-products from corn milling industry, either produced by wet milling or dry-grind processing, getting popular since they have moderate level of protein and lipid as well as no anti-nutritional factors (ANFs) contained in the final product (Regost et al., 1999). The wet-milling process separates the corn into starch, protein, germ, oil and fibre fraction. The corn-endosperm containing protein is separated to make the corn gluten meal (CGM) by-product and contain with high protein (∼60%) and rich in Vitamins B and E (Navarro et al., 2016). In shrimp feed formulation, CGM increasingly used as an ingredient in combination with other plant protein source, such as soybean meal and rendered animal product to reduce the inclusion level of FM (Amaya et al., 2007). However, the solely use of CGM to partially and completely replace FM in the diet cause deterioration in shrimp growth performance. As the levels of CGM increases to replace the use of FM, the shrimp showing significantly lower specific growth rates compared to the control treatment due to lack of lysine and methionine as well as low protein digestibility and reduced palatability. Therefore, innovative advance processing process could be implemented to enhance the nutritional properties of co-products from corn milling process.
One of the advance products from corn protein source, which has better nutritional profile than CGM is corn protein concentrate (CPC). According to Galkanda-Arachchige et al. (2021), CPC is one of the manufactured corn protein source originating from the endosperm after removal of the majority of non-protein components by enzymatic solubilisation of the protein stream obtained from the wet milling process. However, due to the inconsistency results when incorporating CPC in shrimp diet (Zhou et al., 2014), better productivity could be achieved by using more advanced technology to produce ingredients that has superior nutritional profile and closer to the natural diet of Shrimp L. vannamei. Recently, Galkanda-Arachchige et al. (2021) reported about the use of fermented corn protein concentrate (FCPC) as the latest corn-based ingredients with high protein content (∼ 69.10%), low fibre and contain probiotic properties to replace the use of FM in the practical diets of shrimp L. vannamei. However, the effect of complete substitution of FM and partial substitution of FM and poultry by-product meal (PBM) with FCPC with or without krill meal (KM) on the growth and health status of shrimp L. vannamei has not yet been studied. Therefore, the purpose of this study was to determine the effect of FCPC in combination with or without KM to completely and partially replace the FM and/or PBM on growth performance, body composition, total haemocytes, lysozyme activity, intestinal microbiota analysis, tolerance to acute salinity changes and histomorphological condition of Pacific white shrimp Litopenaeus vannamei.
2 | MATERIALS AND METHODS
2.1 | Diet Preparation
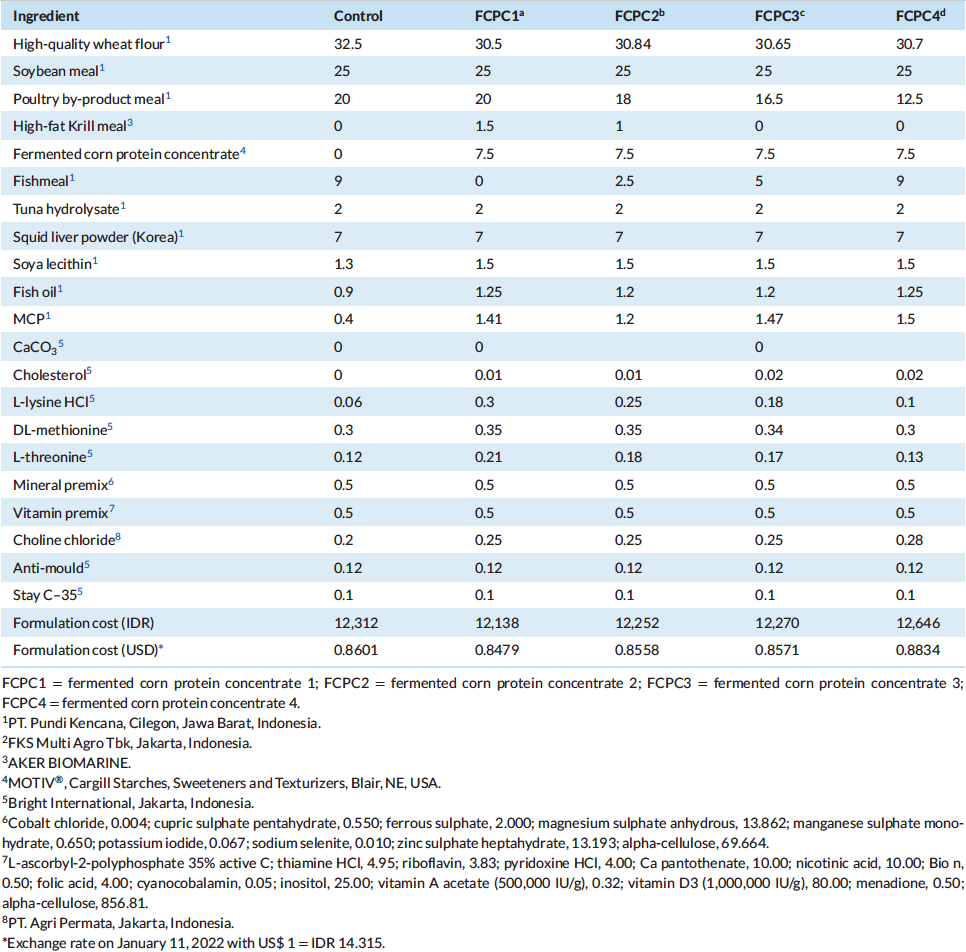
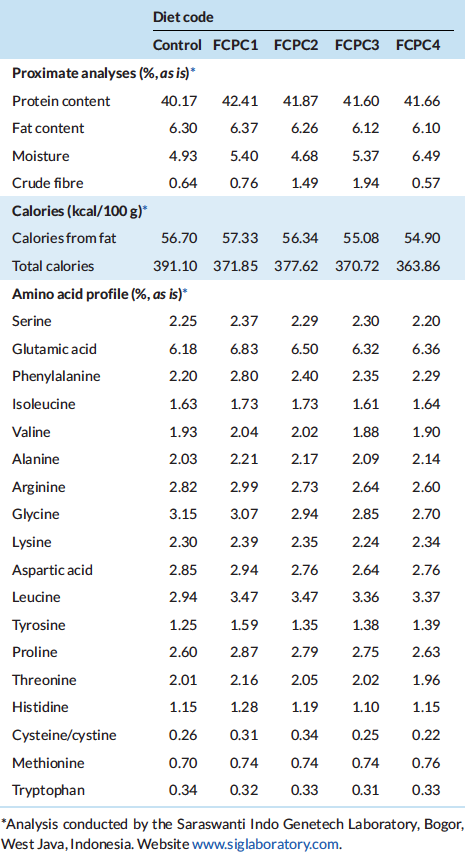
All test diets were formulated to be iso-nitrogenous and iso-lipidic (40% protein and 6% lipid). In this growth trial, basal diet was designed with poultry by-product meal (PBM), soybean meal (SBM), fish meal (FM) and wheat flour (WF) as the primary ingredients. The control diet was designed based on similar approach in Indonesia market by utilising 25%, 20%, 9% and 32.5% of SBM, PBM, FM and WF, respectively. Four experimental diets were formulated to include the use of fermented corn protein concentrate (FCPC, Motiv, Cargill Starches, Sweeteners and Texturizers, Cargill, Inc., Blair, NE, USA) to partially replace the use of animal meal. The first experimental diet was formulated to contain 7.5% FCPC and 1.5% Krill meal (KM) to replace the use of 9% of FM (designated as FCPC 1), second diet was formulated to contain 7.5% FCPC and 1% KM to replace 2% poultry by-product meal (PBM) and 6.5% FM (FCPC2), the third diet contain 7.5% FCPC to replace 3.5% PBM and 4% FM (FCPC3) and the fourth experimental diet was designed to contain 7.5% FCPC to replace 7.5% PBM (FCPC4). All experimental diets were prepared at the Main Center of Mariculture Development of Lampung (Desa Hanura, Lampung, Indonesia) using standard procedures for making shrimp feed. Dry pellets were crumbled, packed in sealed bags, and stored in a freezer until further use. Proximate and amino acids (AA) profile of the diets were analysed at Saraswanti Indo Genetech Laboratory (Bogor, West Java, Indonesia) and summarised in Tables 1 and 2, respectively.
Table 1: Composition (% as is) of diets consisting of fermented corn protein concentrate in commercial diet formulation to replace fish meal and poultry meal and fed to L. vannamei for 60 days
Table 2: Proximate, calories and amino acid (AA) composition (% as is, dry matter basis) of experimental diets utilized in the trial
2.2 | Growth Trial
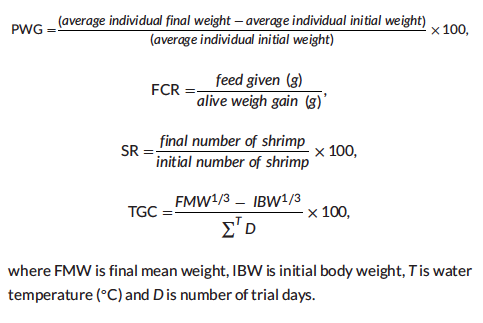
The growth trial was conducted in a semi-closed recirculation system at the Marine Science Techno Park, Diponegoro University (Jepara, Central Java, Indonesia), according to the animal care policy. Water quality was maintained by recirculation through vertical sand filter (Dab Pumps S.p.A., Mestrino, Italy). Dissolved oxygen was maintained near saturation using air stones in each culture tank and the sump tank using a common airline connected to a regenerative blower. A total of 750 Pacific white shrimp post larvae (PL) were obtained from PT. Riz Samudera (Jepara, Central Java, Indonesia) and acclimatised to the culture system. PL were fed with the commercial feed (Feng Li™, Matahari Sakti, Surabaya, East Java, Indonesia) for three weeks until they reached the suitable size. Shrimp with initial mean weight of 1.01 ± 0.04 g were then randomly distributed into 50 tanks with size of 70 × 35 × 40 cm (98 L per aquaria tank) and connected to a common reservoir tank (3000 L). Ten replicate groups of shrimp were administered different types of experimental diets using nutrition research standard protocol for 60 days and fed by hand four times daily, at 07:00, 11:00, 15:00 and 20:00 h. Based on our historic results, feed inputs were pre-programmed assuming the normal growth of shrimp and feed conversion ratio of 1.5. Daily allowances of feed were adjusted based on observed feed consumption, weekly counts of the shrimp and mortality. Uneaten feed, faeces and moults were removed by siphoning the aquaria tank prior to the first feeding. At the conclusion, shrimp were group weighed and final biomass (g), final mean weight (g), survival (%), percentage weight gain (PWG, %), feed conversion ratio and thermal growth coefficient were determined.
2.3 | Water Quality and Growth Sampling
For water quality analysis: pH, dissolved oxygen (DO), water tempera-ture and salinity were monitored twice daily (08:00 and 16:00) using a multi-parameter water checker (HACH, HQ40D, Loveland, CO). Total ammonia-nitrogen (NH3-N) and nitrate-nitrogen (NO3-N) were measured once in a week using Indophenol method and spectrophotometry method (Perkin Elmer, Lambda XLS, USA), respectively. Total counts of bacteria in the water were measured using Tryptic soy agar (TSA; Difco) as a non-selective medium to prepare the culture medium. A subsample of 1 ml water was mixed with 9 ml sterile PBS solution to prepare a 10–1 dilution. Then, subsequent serial dilutions were prepared from 10–2 to 10–5. The spread plate method was used to enumerate the total bacterial counts as CFU ml–1. For total counts of Vibrio sp within the culture environment, 0.1 ml water of water was spread on thiosulphate citrate bile salt (TCBS; Oxoid) agar and measured after 48 h at 28◦C. All equipment and chemicals used in the enumeration process were sterilised properly prior to use. All water quality parameters including total counts of bacteria and Vibrio sp were conducted at the Main Center for Brackishwater Aquaculture Development (Jepara, Central Java, Indonesia). At the end of feeding period, all shrimp were grouped and individually weighed to calculate the final biomass, final mean weight (FMW), percentage of weight gain (PWG), feed conversion ratio (FCR), percentage survival (SR) and thermal unit growth coefficient (TGC) as follows:
2.4 | Body Composition Analysis
Twenty shrimp from each treatment were randomly sampled at the end of the trial and stored at –80◦C for body composition analysis. Prior to proximate, energy and amino acid analysis, dried whole shrimp were rigorously blended and chopped in a mixer according to methods described by Association of Official Analytical Chemists. All parameters were analysed at the Saraswanti Indo Genetech Laboratory (Bogor, West Java, Indonesia).
2.5 | Total Haemocytes Count
At the end of growth trial, haemolymph was sampled from one shrimp per tank or ten shrimp per treatment and total haemocytes count was determined. Haemolymph (100 µl) of individual shrimp was withdrawn from the pleopod base of the second abdominal segment with a sterile 1-ml syringe (25 G × 13 mm needle) containing 100 µl pre-cooled (4◦C) anti-coagulant solution (trisodium citrate 30 mM, NaCl 0.34 M, EDTA 10 mM, pH 7.55,). The haemolymph with anti-coagulant solution was diluted in 150 µl of formaldehyde (4%) and then 20 µl was placed on a haemocytometer (Neubauer) to determine the total haemocyte count (THC) using an optical microscope (Olym-pus, DP72).
2.6 | Lysozyme Activity Analysis
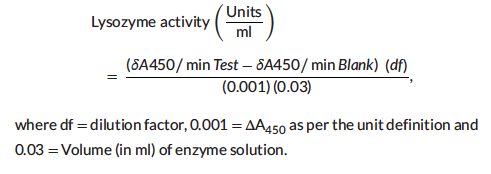
Lysozyme activity was measured by using a lysozyme detection kit (Sigma-Aldrich, Cat. no. LY0100) according to the manufacturer’s instructions. The results of lysozyme activity were defined by the lysis of the Micrococcus lysodeikticus cells. The reactions were conducted at 25◦C and absorbance at 450 nm was measured on the ultravio-let/visible spectrophotometer (Perkin Elmer, Lambda XLS, USA).
2.7 | Hepatopancreas Microbiological Analysis
Tryptic soy agar (TSA; Difco) as a non-selective medium was used for total count of bacteria analysis. The hepatopancreas of shrimp were collected, homogenised and serially diluted with sterile PBS Solution. Suspension (0.1 ml) was spread in triplicate on sterile TSA (with 1%w/v NaCl) to obtain the colony forming unit (CFU) per gram of sample (CFU g–1), which was measured after 24 h at 28◦C.
2.8 | Acute Salinity Change Analysis
At the end of the growth trial, 24 shrimp per treatment were selected for acute salinity change analysis with salinity decreasing from30 ‰ to 0 ‰. The shrimp were distributed into four tanks (6 shrimp tank–1) and left for 60 and 120 min at 0 ppt. The number of survivors were carefully monitored and mortality was recorded after 60 min (P1H = Post 1 h) and 120 min (P2H = Post 2 h) immersed in freshwater (0‰).
2.9 | Histology
Histological analysis was carried out to the hepatopancreas section of the shrimp. The section of approximately 0.5 cm immediately fixed in Bouin’s solution (picric acid-formalin-acetic acid mixture, Ricca Chemical, Arlington, TX, USA) for 20 h at room temperature and then transferred to 70% ethanol solution (VWR, Radnor, PA, USA) until processed by standard histological analysis procedures. Samples were dehydrated through a standard ethanol series to 100%, embedded in paraffin wax and sectioned at 4 µm intervals for staining with haematoxylin-eosin (H&E) stain (Merck, Darmstadt, Germany). Double-blinded evaluations with a grading system of 1 (healthy) to 5 (degraded) were used to evaluate the hepatopancreas condition. Histomorphological images were acquired by using a microscope (Olympus BX41, Olympus Optical Co., Ltd., Tokyo, Japan) with 200 magnification.
2.10 | Statistical Analysis
Growth parameters, proximate composition and amino acids profile of the whole body, total haemocytes counts, lysozyme activities and resistance to the acute salinity changes between dietary groups were analysed using regression and one-way analysis of variance (ANOVA) to determine significant differences among treatments followed by Tukey’s multiple comparison tests to determine the difference between treatment means in each trial. Score data for histomorphological condition of the hepatopancreas of the shrimp were treated as categorical data, tested for normality and homoscedasticity and subsequently analysed using linear regression model. All statistical analyses were conducted using SAS system (V9.4. SAS Institute, Cary, NC, USA).
3 | RESULTS
3.1 | Water Quality
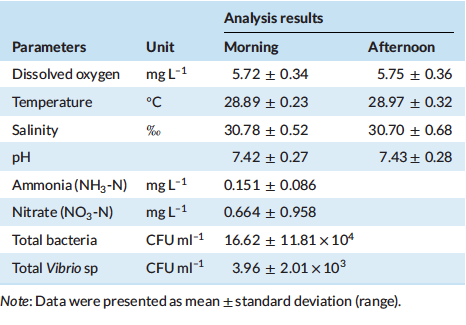
Table 3 indicates that water quality data (mean ± standard deviation), including temperature, D.O., salinity and pH were maintained at 28.89 ± 0.23◦C, 5.72 ± 0.34 mg L–1, 30.78 ± 0.52 ‰ and 7.42 ± 0.27, respectively in the morning and 28.97 ± 0.32◦C, 5.75 ± 0.36 mg L–1, 30.70 ± 0.68 ‰ and 7.43 ± 0.28, respectively, in the afternoon. Total ammonia nitrogen (TAN) and nitrate-nitrogen (NO3-N) were maintained at 0.151 ± 0.086 and 0.664 ± 0.958 mg L–1 during the growth trial and total bacteria and Vibrio sp were in the range of 16.62 ± 11.81 x 104 and 3.96 ± 2.01 x 103 CFU ml–1 during the culture period.
Table 3: Overall water quality measurements during the grow-out phase of the experiment
3.2 | Growth Performance
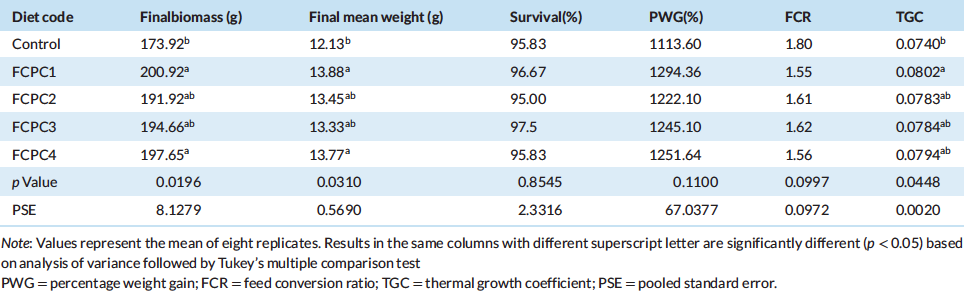
Based on the growth performance and survival of Pacific white shrimp L. Vannamei fed with the experimental diet presented in Table 4, the final biomass (Bio), final mean weight (FMW), and the thermal growth efficient (TGC) were significantly affected by the treatments (p < 0.05). Meanwhile, the treatments did not significantly affect the survival rate (SR), percentage weight gain (PWG) and feed conversion ratio (FCR).
At the end of growth trial, there is a significant increase on growth performance when 7.5% fermented corn protein concentrate (FCPC) and 1.5% of krill meal (KM) were used to replace the utilisation of fish meal (FM) in diet composition. The inclusion of 7.5% FCPC in combination with 9% FM to partially replace the inclusion of poultry by-product meal (PBM) also provide a significant increase in biomass, FMW and TGC of shrimp compared to control treatment. Interestingly, the use of similar level of FCPC in combination with 1% (FCPC2) and 0% KM (FCPC3) to partially replace FM and PBM provide similar growth with shrimp fed with 9% FM or control diet.
Table 4: Growth performance of Pacific white shrimp Litopenaeus vannamei (mean initial weight 4.24 ± 0.03 g) fed experimental diets for 70 days
3.3 | Body Composition Analysis
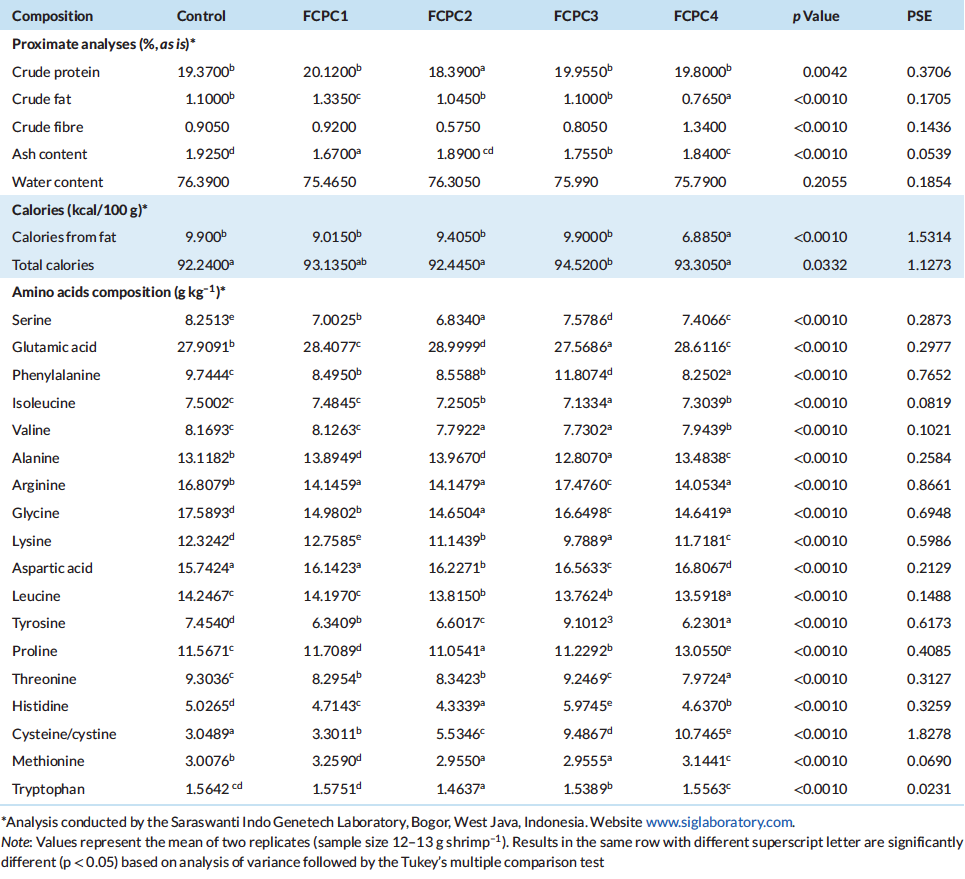
The proximate and amino acids composition of the whole body of shrimp treated with experimental diets are presented in Table 5. At the conclusion of the 60-day culture period, the dietary treatments significantly affect the crude protein, crude fat, crude fibre, ash content and amino acid composition of the shrimp.
Table 5: Proximate and amino acids composition of whole body of shrimp
3.4 | Total Haemocytes, Lysozome Activity and Microbioata Analysis
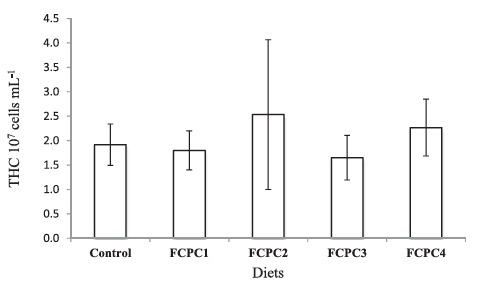
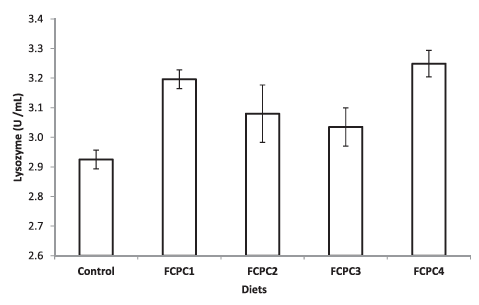
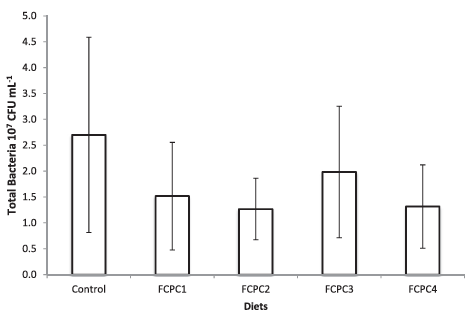
Dietary treatments significantly affect the lysozyme activity of the shrimp but not to the total haemocytes count (Figure 1). The inclusion of 7.5% FCPC with or without KM could enhance the lysozyme activity of shrimp compared to control group (Figure 2). For total count of bacteria in the hepatopancreas of shrimp, statistically, no significant differences were noted among the treatment. However, numerically, total counts of bacteria (CFU g–1) were higher in group of shrimp fed with control feed without any inclusion of FCPC into the formulation.
Figure 1: Total haemocytes count of Pacific white shrimp Litopenaeus Vannamei (107 cell ml–1) at the end of growth trial. Values represent the mean ± standard deviation of eight replicates. Results with different superscript letter are significantly different (p < 0.05) based on analysis of variance followed by Tukey’s multiple comparison test.
Figure 2: Lysozyme activity of Pacific white shrimp Litopenaeus Vannamei (U ml–1) at the end of growth trial. Values represent the mean ± standard deviation of eight replicates. Results with different superscript letter are significantly different (p < 0.05) based on analysis of variance followed by Tukey’s multiple comparison test.
Total bacteria in the culture environment during the growth trial as shown in Table 2 are in the range of 16.62 ± 11.81 × 104 CFU ml–1. Hepatopancreas of shrimp is the major digestive organ and can be drastically affected by pathogens including bacteria. Based on our analysis, the spread plate method did not indicate any significant differences in terms of the number of bacteria in the hepatopancreas of shrimp. However, biologically, we can see that the number of bacteria in shrimp fed with FCPC either with or without KM to partially replace the animal meal provide the lowest number of bacteria compared to the control treatment (Figure 3).
Figure 3: Total number of bacteria (CFU g–1) presence at the hepatopancreas of shrimp. Values represent the mean ± standard deviation of eight replicates.
3.5 | Acute Salinity Change Analysis
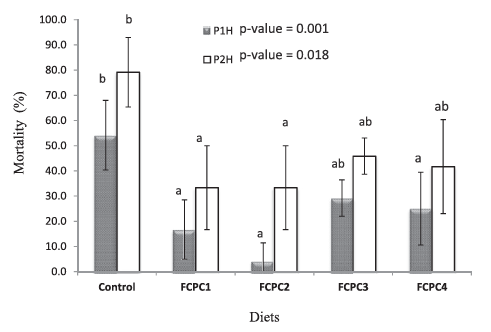
In the next set of experiments, the effect of FCPC on the physiological condition of shrimp L. vannamei was tested in a stress test with zero salinity for 2 h. The results were presented in Figure 4. Here the shrimp fed without FCPC had the highest mortality rates after 1 h post-immersion in freshwater (0 ‰) (P1H= post 1 h) compared to shrimp feds with dietary FCPC. After 2 h (P2H), the mortality rate in all group continuous to increase including the shrimp fed with FCPC. In general, at P2H, the lowest mortality was found in the group of FCPC1 and FCPC2, while statistically there is no significant difference in terms of mortality rates between control, FCPC3 and FCPC4 group (p = 0.018).
Figure 4: Mortality (%) of Litopenaeus vannamei over 1 h post-stress (P1H) and 2 h post-stress (P2H) in freshwater (0 ‰). Values represent the mean ± standard deviation of four replicates. Results with different superscript letter are significantly different (p < 0.05) based on analysis of variance followed by Tukey’s multiple comparison test.
3.6 | Histomorphological Condition at the Hepatopancreas of Shrimp
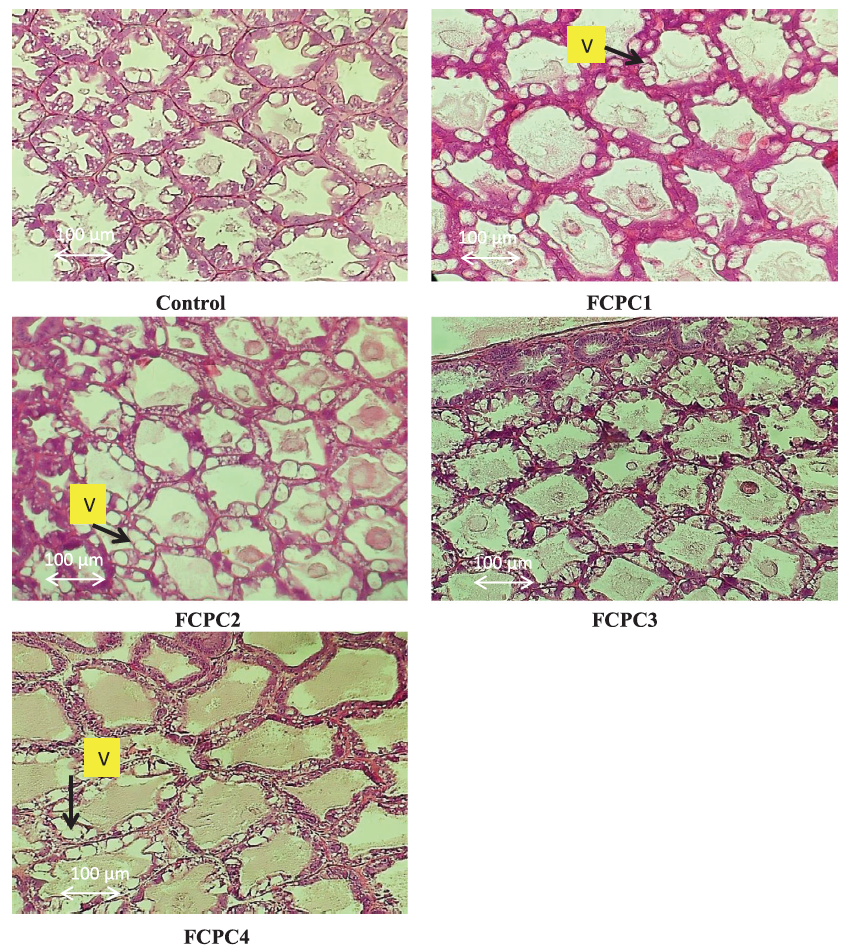
Representative histological images of shrimp L. vannamei hepatopancreas is shown in Figure 5. After being fed with the experimental diets for 60 days, shrimp exhibited an excess of fat in several vacuolated cells. This vacuolation and excess fat condition were seen in shrimp fed with FCPC1, FCPC 2 and FCPC 4.
Figure 5: Representative histomorphological images from haematoxylin and eosin-stained sections of the hepatopancreas of Pacific white shrimp Litopenaeus vannamei fed for 60 days with the experimental diet. Measurements of the vacuolation (V) were performed with 200× magnification. FCPC1, 7.5% FCPC+ 1.5% KM; FCPC2, FCPC 7.5%+ 1% KM; FCPC3, 7.5% FCPC to replace 3.5% PBM and 4% FM; and FCPC4, 7.5% FCPC to replace 7.5% PBM.
4 | DISCUSSION
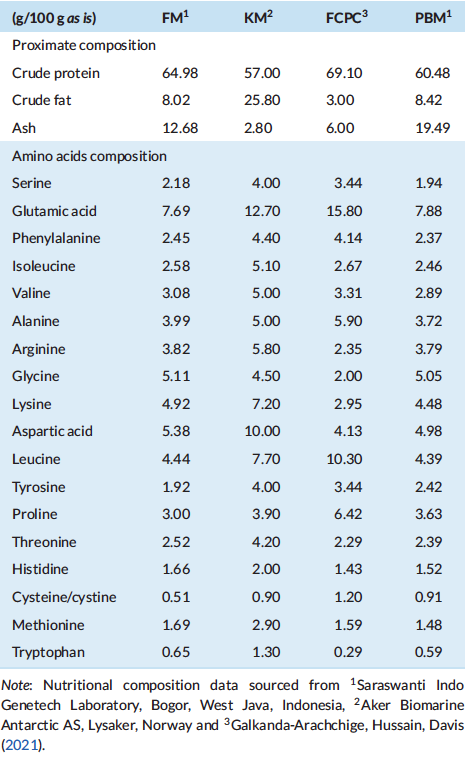
In the present study, significant differences were observed in final biomass, final mean weight and the thermal growth coefficient among the treatments. The differences could be attributed to the addition of fermented corn protein concentrate (FCPC, Motiv, Cargill Starches, Sweeteners and Texturizers, Blair, NE, USA) in combination with proper inclusion levels of animal meal in shrimp feed formulation. The highest growth rate was shown in shrimp fed with 7.5% FCPC in combination with 1.5% krill meal (KM) to completely replace fish meal (FM) (FCPC1) and 7.5% FCPC in combination with 9% FM (FCPC4) to significantly reduce the inclusion levels of poultry by-product meal (PBM). Recently, Galkanda-Arachchige et al. (2021) reported the efficacy of FCPC as the latest advance products of corn-based ingredients to maintain the growth, FCR and survival of shrimp L. vannamei with gradual inclusion from 4% to 15% to partially and completely replace the use of FM in the diet. The authors suggest that the better nutritional profile with no deficiencies in amino acids (Table 6) could be the reason for similar growth performance among shrimp fed with the experimental diets and the basal diet (Galkanda-Arachchige et al., 2021).
Interesting to note that in our present study, the use of 7.5% of FCPC in combination with 2.5% FM and 1% KM (FCPC2) and 7.5% FCPC in combination with 5% FM (FCPC3) to partially replace PBM in the diet, numerically, also provide better growth compared to the control group. Considering the nutritional profile of FCPC, the results are in very close agreement with Ray et al. (2020) where proper blending of concentrated plant-protein sources with low inclusion levels of animal meal could provide better growth compared to the sole use of FM. Using better replacement strategies that consider the nutritional profile of the ingredients would result in important savings in feed cost. In the condition of the present study, partial replacement of FM with FCPC with and without KM could reduce the formulation cost of the practical diet (Table 1). Despite no statistical differences, the FCR in group of shrimp fed with 7.5% FCPC either in combination with KM or FM was better than shrimp fed with 9% FM without addition of FCPC (control). Looking at the study results from Galkanda-Arachchige et al. (2021), gradual increase of FCPC to partially and completely replace FM in practical diets of vannamei had no significant effects on FCR. The feasible reasons with lower FCR in group of vannamei treated with FCPC in our study could be attributed to the fermentation process that enhances the nutritional profile and digestion of the corn products.
In a situation where shrimp right now facing serious problems with infectious diseases and lack of true adaptive immune system (Kulka-rni et al., 2021), the use of feed with healthy dietary ingredients that could enhance the innate defence mechanisms based on non-specific immune system in response to invasion of pathogen is required. As FCPC has been reported to contain probiotic properties that could proactively boost the disease resistance in shrimp (Galkanda-Arachchige et al., 2021), we have investigated the total haemocytes count (THC), lysozyme activity and the number of bacteria presence at the hepatopancreas of shrimp. In our study, we found no significant differences in the number of THC among the dietary treatment. This is in agreement with Galkanda-Arachchige et al. (2021) where no significant differences were noted in the number of THC among the shrimp fed with FCPC to partially and completely replace the inclusion of FM in shrimp diet. However, we noticed significant differences in the lysozyme activity of shrimp with group of shrimp treated with FCPC showed a higher quantitative number compared to the control group. According to Novriadi et al. (2021), lysozyme activity which is produced by shrimp haemocytes act as an antimicrobial protein and become one of the important element to enhance the non-specific immune system in shrimp against pathogen. Activity of lysozyme could be increase with the increase of haemocytes concentrations, but it is not always the case. Study from Burge et al. (2007) revealed that in the case of infection, haemocytes could be recruited to the site of injection early during the course of immune response thus reducing the number of haemo-cytes in circulation. Recently, the addition of hydrolysable tannins as the functional ingredients in the practical diets of vannamei also pro-duce significant increases in lysozyme activity in shrimp treated with tannins compared to the control treatment but not for the THC (Novri-adi et al., 2021). Based on this situation, time series analysis to quan-tify the lysozyme expression and haemocytes counts will be necessary to provide better understanding on how the addition of FCPC could enhance the non-specific immune system in shrimp.
Total bacteria count (CFU g–1) has been used to assess the hepatopancreas condition in shrimp. The present study showed that, numerically, the number of total bacteria in the hepatopancreas treated with FCPC is lower in the range of 1.267 ± 0.593 to 1.983 ± 1.269 × 107 CFU g–1 than the control treatment (2.700 ± 1.887 CFU g–1). However, no external pathology was observed in the control treatment and the hepatopancreas looked normal in size, colour and texture. In addition, during the acute stress test to distinguish between healthy and weak shrimp by immersing the shrimp in water with sudden changes in salinity from 30.78 ± 0.52 ‰ to 0 ‰, the mortality rate of shrimp was higher in control treatment compared to the shrimp treated with FCPC either after 1 or 2 h immersion in 0 ‰ water. According to Castille and Lawrence (1981), juvenile and adult penaeid shrimp were capable both hypo- and hyper-osmotic regulation. However, mortality may occur due to inadequate handling procedures and nutritional deficiencies that lead to ineffective regulation of osmotic balance and resistance to salinity changes in shrimp (Liu et al., 2007). Study from Liu et al. (2007) found that proper inclusion level of vitamin E in shrimp diet could enhance the resistance of shrimp to acute changes in salinity. Moreover, Zheng et al. (2017) reported that the addition of Lactobacillus plantarus significantly improved the resistance of L. vannamei against the stress of acute low salinity, as indicated by higher survival rates and increase on Prophenoloxidase (ProPO) transcript levels. Since FCPC could enhance the non-specific immune system of shrimp due to the probiotic properties contain in the final product (Galkanda-Arachchige et al., 2021), better survival is possible through the benefits derived from the FCPC.
In shrimp, hepatopancreas play significant role in synthesis and secretion of digestive enzymes and immune molecules, absorption of nutrients and deposition of lipids (Al-Mohanna & Nott, 1989; Dall et al., 1990). In our study, after being fed with dietary treatments, shrimp exhibited minor histological alterations in the hepatopancreas with an excess of fat in several vacuolated cells, especially in shrimp fed with FCPC 1, FCPC 2 and FCPC 4. According to Zhao et al. (2017), vacuolated cells can be regarded as reliable biomarkers of toxic injury and disrupt the detoxification functions of the hepatopancreas. Knowledge on the inclusion effect of FCPC in the hepatopancreas of shrimp is limited. The excess of fat in vacuolated cells in the hepatopancreas of shrimp could also be due to the use of KM contained with 25.80% crude fat level (Table 6) into the practical diet, and become the highest among the ingredients used in this study. Interestingly, the vacuolated cells only occur in hepatopancreas of shrimp treated with 1% KM (FCPC2) and 9% FM (FCPC4), but not in shrimp fed with FCPC with low inclusion levels of FM (FCPC3). Since there were no significant effects to the growth of shrimp treated with FCPC compared to the control treatment, further study is needed to confirm the actual effect of FCPC to the hepatopancreas condition and growth of the shrimp until reach the consumption size.
Table 6: Proximate and amino acid composition (% as is) of fish meal (FM), krill meal (KM), fermented corn protein concentrate (FCPC) and poultry by-product meal (PBM)
5 | CONCLUSION
The results of this study indicated that the inclusion of fermented corn protein concentrate (FCPC) into the practical diets of vannamei has significantly improved growth performance and lysozyme activity of Pacific white shrimp Litopenaeus vannamei. Inclusion of FCPC improved the resistance of L. vannamei against acute low salinity stress as well as low number of bacteria present in the hepatopancreas. The use of FCPC in combination with KM and FM causes an excess fat in the vacuolated cells of hepatopancreas in shrimp. However, the condition did not significantly affect the growth performance of the shrimp. Nevertheless, further investigations are needed to clarify the mode of action of FCPC to the THC and lysozyme activity expression genes in time series analysis as well as to provide better understanding on the inclusion effect of FCPC to the hepatopancreas condition and growth performance of the shrimp in long-term growth trial using commercial shrimp production system.
ACKNOWLEDGEMENTS
The work was supported by the grants from Cargill Starches, Sweeteners and Texturizers. Blair. NE. USA. Special thanks to Aldy Eka Wahyudi from Jakarta Technical University of Fisheries and Aksan from Diponegoro University for their helps during the research program. The authors would also like to extend the gratitude to those who have taken the time to critically review this manuscript as well as those who helped in supporting this research. Mention of trademark or proprietary product does not constitute an endorsement of the product and does not imply its approval to the exclusion of other products that may also be suitable. Motiv, Cargill Starches, Sweeteners and Texturizers, Blair, NE, USA provided the fermented corn protein concentrate (FCPC) as well as funding for this study.
AUTHOR CONTRIBUTIONS
Romi Novriadi: conceptualisation; data curation; formal analysis; investigation; methodology; resources; supervision; validation; writing – original draft; writing – review & editing. Vivi Endar Herawati: conceptualisation; data curation; formal analysis; investigation; methodology; supervision; validation. Slamet Budi Prayitno: conceptualisation; data curation; formal analysis; investigation; methodology; resources; supervision. Seto Windarto: conceptualisation; data curation; formal analysis; investigation; methodology; supervision. Keith Mertz: conceptualisation; data curation; formal analysis; supervision; visualisation; writing – review & editing. Nguyen Duy Hoa: conceptualisation; data curation; formal analysis; investigation; methodology; supervision; validation; visualisation.
CONFLICT OF INTEREST
Keith Meirtz and Nguyen Duy Hoa are employed by Cargill Incorporated. The rest of the authors state no conflict of interest.
DATA AVAILBILITY STATEMENT
Data associated with this manuscript entitled ‘Effect of fermented protein concentrate on growth performance, haemocytes counts, histological structure of hepatopancreas and intestinal condition of Pacific white shrimp Litopenaeus vannamei’ are available at Dr. Romi Novriadi research group and could be accessed based on the permission of Dr. Romi Novriadi.
PEER REVIEW
The peer review history for this article is available at https://publons. com/publon/10.1002/aff2.37.
ORCID
Romi Novriadi https://orcid.org/0000-0002-1904-5781
REFERENCES:
Al-Mohanna, S. & Nott, J. (1989) Functional cytology of the hepatopancreas of Penaeus semisulcatus (Crustacea: Decapoda) during the moult cycle. Marine Biology, 101, 535–544.
Amaya, E.A., Davis, D.A. & Rouse, D.B. (2007) Replacement of fish meal in practical diets for the Pacificwhite shrimp (Litopenaeus vannamei) reared under pond conditions. Aquaculture, 262, 393–401.
Arantes, R., Schveitzer, R., Magnotti, C., Lapa, K.R. & Vinatea, L. (2017) A comparison between water exchange and settling tank as a method for suspended solids management in intensive biofloc technology systems: Effects on shrimp (Litopenaeus vannamei) performance,water quality and water use. Aquaculture Research, 48, 1478–1490.
Argue, B.J., Arce, S.M., Lotz, J.M., & Moss, S.M. (2002) Selective breeding of Pacific white shrimp (Litopenaeus vannamei) for growth and resistance to Taura Syndrome Virus. Aquaculture. 204, 447–460.
Ariadi, H., Fadjar, M. & Mahmudi, M. (2019) Financial feasibility analysis of shrimp Vannamei (Litopenaeus vannamei) culture in intensive aquaculture system with low salinity. ECSOFiM (Economic and Social of Fisheries and Marine Journal), 7, 95–108.
Burge, E.J., Madigan, D.J., Burnett, L.E., & Burnett, K.G. (2007) Lysozyme gene expression by hemocytes of Pacific white shrimp, Litopenaeus vannamei, after injection with Vibrio. Fish & Shellfish Immunology, 22, 327–339.
Castille Jr, F.L. & Lawrence, A.L. (1981) The effect of salinity on the osmotic, sodium and chloride concentrations in the hemolymph of euryhaline shrimp of the genus Penaeus. Comparative Biochemistry and Physiology Part A: Physiology, 68, 75–80.
Chithambaran, S., Harbi, M., Broom, M., Khobrani, K., Ahmad,O., Fattani,H., Sofyani, A. & Ayaril, N. (2017) Green water technology for the production of Pacific white shrimp Penaeus vannamei (Boone, 1931). Indian Journal of Fisheries, 64(3), 63656–63607.
Colombo, S.M. & Turchini, G. (2021). ’Aquafeed 3.0’: Creating a more resilient aquaculture industry with a circular bioeconomy framework. Reviews in Aquaculture, 13.
Dall, W., Hill, B., Rothlisberg, P.C. & Sharples, D. (1990) The biology of the Penaeidae. Advances in Marine Biology, 27, iii-vi, 1–489.
Davis, D.A. & Arnold, C. (2000) Replacement of fish meal in practical diets for the Pacific white shrimp, Litopenaeus vannamei. Aquaculture. 185, 291–298.
FAO (2020) The State ofWorld Fisheries and Aquaculture, 2018. Food and Agriculture Organization of the United Nations.
Galkanda-Arachchige, H.S., Hussain, A.S. & Davis, D.A. (2021) Fermented corn protein concentrate to replace fish meal in practical diets for Pacific white shrimp Litopenaeus vannamei. Aquaculture Nutrition, 27, 1640– 1649.
Guo, J., Reis, J., Salze, G., Rhodes, M., Tilton, S., & Davis, D.A. (2019) Using high protein distiller’s dried grain product to replace corn protein concentrate and fish meal in practical diets for the Pacific white shrimp Litopenaeus vannamei. Journal of the World Aquaculture Society, 50, 983–992.
Jackson, A. (2012) Fish meal and fish oil and its role in sustainable aquaculture. International Aquafeed, 15, 18–21.
Karim, M., Sarwer, R., Phillips, M. & Belton, B. (2014) Profitability and adoption of improved shrimp farming technologies in the aquatic agricultural systems of southwestern Bangladesh. Aquaculture, 428, 61–70.
Kulkarni, A., Krishnan, S., Anand, D., Kokkattunivarthil Uthaman, S., Otta, S.K., Karunasagar, I. & Kooloth Valappil, R. (2021) Immune responses and immunoprotection in crustaceans with special reference to shrimp. Reviews in Aquaculture, 13, 431–459.
Lebel, L., Mungkung, R., Gheewala, S.H., & Lebel, P. (2010) Innovation cycles, niches and sustainability in the shrimp aquaculture industry in Thailand. Environmental Science & Policy, 13, 291–302.
Lebel, L., Garden, P., Luers, A., Manuel-Navarrete, D. & Giap, D.H. (2016) Knowledge and innovation relationships in the shrimp industry in Thailand and Mexico. Proceedings of the National Academy of Sciences, 113, 4585–4590.
Lightner, D.V. (2005) Biosecurity in shrimp farming: Pathogen exclusion through use of SPF stock and routine surveillance. Journal of the World Aquaculture Society, 36, 229–248.
Lim, C. & Dominy, W. (1990) Evaluation of soybean meal as a replacement for marine animal protein in diets for shrimp (Penaeus vannamei). Aquaculture, 87, 53–63.
Liu, Y., Wang, W.-N., Wang, A.-L., Wang, J.-M. & Sun, R.-Y. (2007) Effects of dietary vitamin E supplementation on antioxidant enzyme activities in Litopenaeus vannamei (Boone, 1931) exposed to acute salinity changes. Aquaculture, 265, 351–358.
Martinez-Cordero, F.J. & Leung, P. (2004) Sustainable aquaculture and producer performance: Measurement of environmentally adjusted productivity and efficiency of a sample of shrimp farms in Mexico. Aquaculture, 241, 249–268.
Moss, S.M., Moss, D.R., Arce, S.M., Lightner, D.V. & Lotz, J.M. (2012) The role of selective breeding and biosecurity in the prevention of disease in penaeid shrimp aquaculture. Journal of Invertebrate Pathology, 110, 247–250.
Navarro, S.L., Capellini, M.C., Aracava, K.K. & Rodrigues, C.E. (2016) Corn germ-bran oils extracted with alcoholic solvents: Extraction yield, oil composition and evaluation of protein solubility of defatted meal. Food and Bioproducts Processing, 100, 185–194.
Novriadi, R. & Davis, D.A. (2018) Research update: Development of plant-based diets for Florida pompano Trachinotus carolinus. Aquacultura Indonesiana, 19, 47–56.
Novriadi, R., Fadhilah, R., Wahyudi, A.E. & Trullàs, C. (2021) Effects of hydrolysable tannins on the growth performance, total haemocyte counts and lysozyme activity of Pacific white leg shrimp Litopenaeus vannamei. Aquaculture Reports, 21, 100796.
Qiu, X., Tian, H. & Davis, D.A. (2017) Evaluation of a high protein distiller’s dried grains product as a protein source in practical diets for Pacific white shrimp Litopenaeus vannamei. Aquaculture, 480, 1–10.
Ray, G.W., Liang, D., Yang, Q., Tan, B., Dong, X., Chi, S., Liu, H., Zhang, S., & Rimei, L., (2020) Effects of replacing fish meal with dietary soybean protein concentrate (SPC) on growth, serum biochemical indices, and antioxidative functions for juvenile shrimp Litopenaeus vannamei. Aquaculture, 516, 734630.
Rego, M.A.S., Sabbag, O.J., Soares, R. & Peixoto, S. (2017). Financial viability of inserting the biofloc technology in a marine shrimp Litopenaeus vannamei farm: A case study in the state of Pernambuco, Brazil. Aquaculture international, 25, 473–483.
Regost, C., Arzel, J. & Kaushik, S. (1999) Partial or total replacement of fish meal by corn gluten meal in diet for turbot (Psetta maxima). Aquaculture, 180, 99–117.
Reis, J., Novriadi, R., Swanepoel, A., Jingping, G., Rhodes, M. & Davis, D.A. (2020) Optimizing feed automation: Improving timer-feeders and on demand systems in semi-intensive pond culture of shrimp Litopenaeus vannamei. Aquaculture, 519, 734759.
Rhodes, M.A., Yu, D., Zhou, Y. & Allen Davis, D. (2015) Use of lipid-extracted distillers dried grain with solubles (DDGS) in diets for Pacific white shrimp. North American Journal of Aquaculture, 77, 539–546.
Silva, J.F., Hamilton, S., Rocha, J.V., Borie, A., Travassos, P., Soares, R. & Peixoto, S. (2019) Acoustic characterization of feeding activity of Litopenaeus vannamei in captivity. Aquaculture, 501, 76–81.
Sookying, D., & Davis, D.A. (2011). Pond production of Pacific white shrimp (Litopenaeus vannamei) fed high levels of soybean meal in various combinations. Aquaculture, 319, 141–149.
Sookying, D., Davis,D.&SollerDiasDa Silva, F. (2013)Areview of the development and application of soybean-based diets for Pacific white shrimp Litopenaeus vannamei. Aquaculture Nutrition, 19, 441–448.
Wang, Q., Yu, Y., Zhang, Q., Zhang, X., Yuan, J., Huang, H., Xiang, J. & Li, F. (2019) A novel candidate gene associated with body weight in the Pacific White Shrimp Litopenaeus vannamei. Frontiers in genetics, 10, 520.
Xiong, J., Zhao, Y., Gao, Y., Xie, D., Zhang, B. & Chen, X. (2011) Advances in breeding improved varieties of Litopenaeus vannamei. Journal of Southern Agriculture, 42, 556–561.
Zhao, W., Wang, L., Liu, M., Jiang, K., Wang, M., Yang, G., Qi, C. & Wang, B. (2017) Transcriptome, antioxidant enzyme activity and histopathology analysis of hepatopancreas from the white shrimp Litopenaeus vannamei fed with aflatoxin B1 (AFB1). Developmental & Comparative Immunology, 74, 69–81.
Zheng, X., Duan, Y., Dong, H., & Zhang, J. (2017) Effects of dietary Lactobacillus plantarum in different treatments on growth performance and immune gene expression of white shrimp Litopenaeus vannamei under normal condition and stress of acute low salinity. Fish & shellfish immunology, 62, 195–201.
Zhou, Y., Fang, X., Oliveira, K., Yu, D., Cook, R.L., Rhodes, M.A. & Davis, D.A. (2014) Growth response of corn protein concentrate in practical diets for Pacific white shrimp Litopenaeus vannamei,World Aquaculture Society Meetings, Aquaculture American, At Seattle,WA.
HOW TO CITE THIS ARTICLE:
Novriadi, R., Herawati, V.E., Prayitno, S.B.,Windarto, S., Mertz, K. & Nguyen Duy, H. (2022) Effect of fermented corn protein concentrate on growth performance, haemocyte counts, histological structure of hepatopancreas and intestinal condition of pacific white shrimp Litopenaeus vannamei. Aquaculture Fish and Fisheries, 1–12. https://doi.org/10.1002/aff2.37
Cargill Branded Feed creates proprietary feed ingredients to improve digestive health and performance for production animals in the beef, dairy, aquaculture and pet food markets. Branded Feed is a segment of Cargill Starches, Sweeteners & Texturizers (CSST).